Therapeutic drug monitoring (TDM) plays a significant role in clinical decision-making and is rapidly
becoming an essential and specialized element of patient treatment.
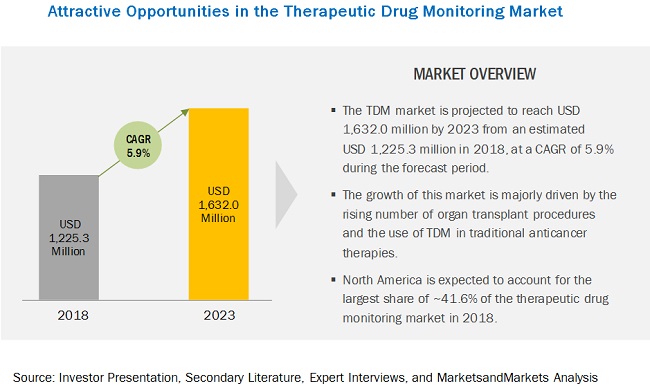
TDM is the branch of clinical chemistry that deals with the measurement of specific drugs at designated intervals to maintain a constant concentration in a patient’s bloodstream, thereby optimizing individual dosage regimens.
The apDia ELISA’s uses highly specific monoclonal antibodies developed at the University of Leuven, Belgium (KU Leuven).
We have ELISA’s for :
- IFX ( Remicade® and biosimilars) and anti-IFX
- ADM ( Humira® and biosimilars) and anti-ADM
- VDZ ( Entyvio®)
- GLM (Simponi ®)
- UST ( Stelara® )
Advantages of the apDia-ELISA kits:
- ELISA testing
- Validated by KU Leuven
- Ready to use reagents
- Validated for automated ELISA systems f.i. Dynex automate DS2
- Reagents commonly used in the TDM assays – Sample Diluent, Wash Solution, Chromogen Solution and Stop Solution – are interchangeable across the TDM assays
- The different apDia TDM assays for Biologicals (IFX-ADM-GLM-VDZ-UST) can be combined on a microtiterplate

