EndomKIT (REF. 780001)
EndomKIT is an IVD test intended for use in women with suspected endometriosis in conjunction with other available clinical information to aid in the diagnosis of endometriosis. The ELISA kit contains 48 tests for measuring brain-derived neurotrophic factor (BDNF) and cancer antigen 125 (CA125), combined with a questionnaire for defining 6 clinical variables.

Figure: ASRM classification of endometriosis I–IV stages. Pink arrows indicate superficial implants; purple arrows indicate dense adhesions; blue arrows indicate filmy adhesions(10)
What is Endometriosis?
Endometriosis is a chronic, debilitating disease defined as endometrial-like tissue outside the uterine cavity that leads to an oestrogen-dependent chronic inflammatory state, frequently associated with dysmenorrhea, non-menstrual pelvic pain and infertility(1-3). There are three clinical manifestations of endometriosis: superficial endometriosis, ovarian endometrioma, deeply infiltrating endometriosis (DIE).
Endometriosis can be classified based on a point system, into one of four progressive stages (see figure) depending on location, extent, and depth of endometriosis implants; presence and severity of adhesions; and presence and size of ovarian endometriomas. Most women have minimal or mild endometriosis, which is characterized by superficial implants and mild adhesions. Moderate and severe endometriosis are characterized by chocolate cysts in the ovaries and more severe adhesions. Although the stage of endometriosis does not always correlate with the presence or severity of symptoms, infertility is very likely with stage IV endometriosis(9).
It affects 5-10% of women of reproductive age. The prevalence of the disease is 50% among women with chronic pelvic pain and 24% among infertile women(4,6). Despite this high prevalence, disease recognition is inadequate and diagnosis time ranges from 4 to 11 years, with 65% of women being initially misdiagnosed(8).
Current method of diagnosis
At present, endometriosis is presumptively diagnosed as a result of patient symptomatology and imaging techniques (transvaginal ultrasound and magnetic resonance imaging). However, confirmation of the disease can only be obtained by a laparoscopic surgery followed by histopathological evaluation of the lesions(7). Although being the gold standard for diagnosing the disease, laparoscopy being invasive contributes to this delay in diagnosis(5,6). The diagnosis of women presenting only with superficial lesions is of special interest due to the limited value of existing imaging techniques for their identification, possibly leading to underdiagnosis and numerous misdiagnoses(6).
Earlier diagnosis with a simple assay could help provide timely treatment of young patients, thereby avoiding severe complications and improving quality of life while facilitating clinical management (medical treatment, surgery, assisted reproductive technologies).

The EndomKIT
The IVD test consists of a manual quantitative ELISA test kit for the determination of human venous serum BDNF and CA125 to be used in conjunction with a data treatment algorithm hosted in a diagnostic medical software. The input variables for the data treatment algorithm are the serum concentrations of BDNF and CA125 in combination with patient’s clinical information. The IVD test provides a qualitative, “positive” or “negative”, result.
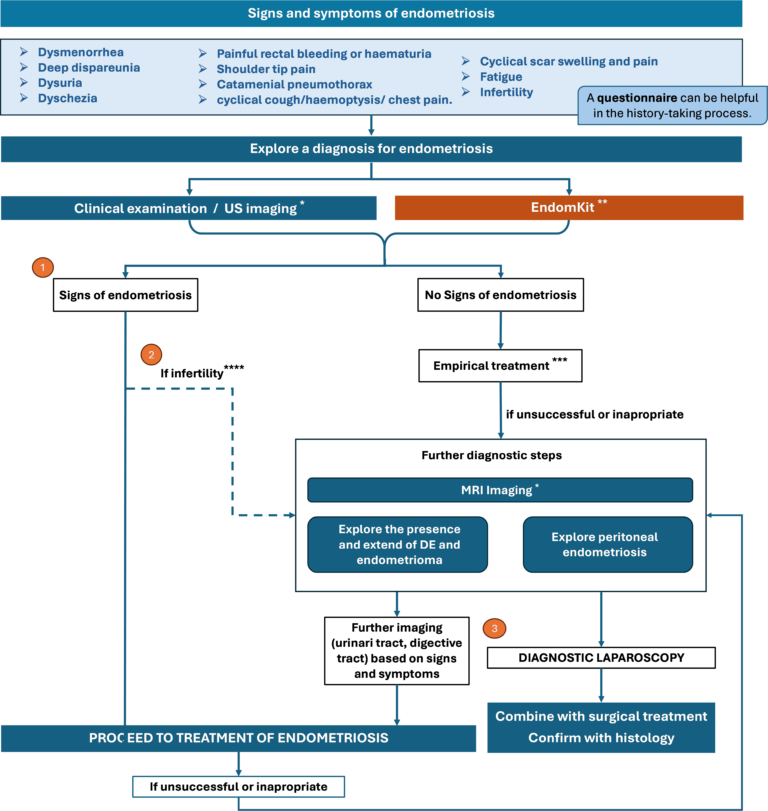
A testing strategy based on the use of the EndomKIT:


The Clinical performance validation study
The validation study involved computing algorithm scores and corresponding outcomes using the IVD test software. A positive diagnosis was assigned when the score exceeded the predetermined cut-off, while a negative diagnosis was given when the score was below the cut-off.
The diagnostic performance of the IVD test had a diagnostic sensitivity (after weighting for disease stages) of 46.2% (95% CI: 25.5–66.8%) and a specificity of 100% (95% CI: 86.7–100%). The accuracy was 64.1% (95% CI: 50.4–77.8%), and the AUC was 0.758 (95% CI: 0.650–0.867). A good specificity was the primary objective because this assay is primarily intended to aid in identifying individuals with endometriosis. Interestingly, even in the presence of various confounding medical conditions, the diagnostic performance is not significantly affected(6).
VALIDATION STUDIES
EndomKIT
Procedure has been validated on the open ELISA DYNEX® DS2 instrument.
instructions for use (IFU) available upon request
References:
(1) Rogers, P. A., et al. (2009). Priorities for endometriosis research: Recommendations from an international consensus workshop. Reproductive Sciences, 16(4), 335–346
(2) Giudice, L. C. (2010). Clinical practice: Endometriosis. The New England Journal of Medicine, 362(25), 2389–2398
(3) Zondervan, K. T., et al. (2018). Endometriosis. Nature Reviews Disease Primers, 4, 9
(4) Parazzini, F., et al. (2020). The frequency of endometriosis in the general and selected populations: A systematic review. Journal of Endometriosis and Pelvic Pain Disorders, 12(3-4), 176-189
(5) Albee, R. B. Jr, Sinervo, K., & Fisher, D. T. (2008). Laparoscopic excision of lesions suggestive of endometriosis or otherwise atypical in appearance: Relationship between visual findings and final histologic diagnosis. Journal of Minimally Invasive Gynecology, 15(1), 32–37
(6) Herranz-Blanco, B., et al. (2023). Development and validation of an endometriosis diagnostic method based on serum biomarkers and clinical variables. Biomolecules, 13, 1052
(7) Exeltis. (2024). Diagnostic Model for the Detection of Endometriosis
(8) Agarwal, S. K., et al. (2019). Clinical diagnosis of endometriosis: A call to action. American Journal of Obstetrics and Gynecology, 220(4), 354.e1–354.e12
(9) American Society for Reproductive Medicine. (2016). Endometriosis: A guide for patients. American Society for Reproductive Medicine under the direction of the Patient Education Committee and the Publications Committee
(10) Di Renzo, L., et al. (2023). Endometriosis treatment: Role of natural polyphenols as anti-inflammatory agents.
(11) Daoud, E., Archer, D., & Herranz-Blanco, B. (2024). Validation of an in vitro diagnostic test for endometriosis: Impact of confounding medical conditions and lesion location. medRxiv.
ORDER NOW
- Call or mail us now.
- You get in touch directly with your contact person.
- You get a concrete answer for the next step.


